You are viewing the article What is hard water? Recognizing signs and effective ways to soften hard water at Lassho.edu.vn you can quickly access the necessary information in the table of contents of the article below.
Hard water is water with a high mineral content, formed when groundwater seeps through layers of limestone, chalk, or gypsum. In this article, let’s find out what hard water is, as well as telltale signs and effective ways to soften hard water!
See now the core of the water purifier is on SHOCKING discount
What is hard water?
Hard water is water that contains a high content of dissolved minerals in the form of ions, mainly calcium cations (Ca2+) and magnesium (Mg2+) exceeding the permissible level (over 300mg/liter).

Causes of hard water formation
Hard water is created when water flowing from a source or groundwater flows through layers of limestone, gypsum, or chalk. These are rocks that contain large amounts of calcium and magnesium ions in the form of carbonate, hydrocarbonate, and sulfate compounds. During that process, small amounts of minerals are dissolved and retained by the water, imparting hardness to the water.
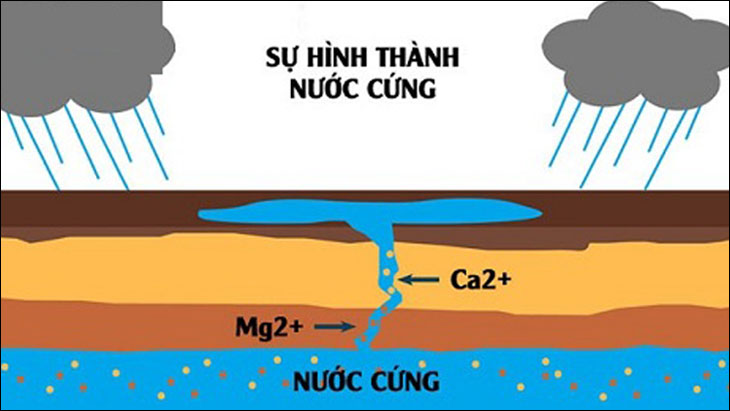
Groundwater sources often have high hardness by the process of dissolving Mg2+, Ca2+ ions present in the composition of limestone sediments… when passing through the rock layers, thereby increasing the hardness in the water. Besides, water in ponds, rivers and streams can also increase hardness due to this reason.
Components in hard water
The main components in hard water are dissolved minerals in the form of ions, which are mainly calcium cations (Ca2+) and magnesium (Mg2+). In addition, hard water may also contain small amounts of iron ions and other metal ions such as strontium, aluminum, barium, manganese, zinc, etc.

Water hardness levels
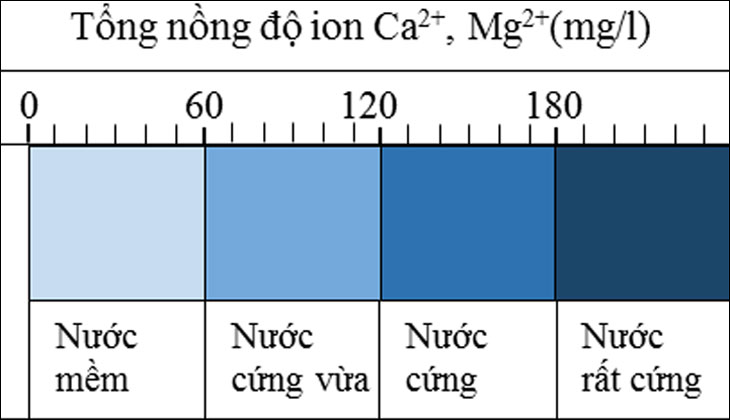
Based on the index of total concentration of Ca2+ and Mg2+ ions, water hardness is divided into 4 different levels, namely:
- From 0 to 60 mg/liter: Soft water
- From 60 – 120 mg/liter: Moderately hard water
- From 121 – 180 mg/liter: Hard water
- Over 180 mg/liter: Very hard water
Signs of hard water
In fact, there are many ways to identify hard water, specifically you can refer to some of the signs below to see if the water you are using is hard water.
- On faucets, showers are rusty, build up stains, pipes and faucets are easily clogged.
- After a period of use, metal cooking utensils such as pots, pans, … and especially kettles will appear deposits or white patches on the bottom.

- Detergent or other detergents are difficult to dissolve in water, at least foam, resulting in clothes and fabric items after washing still have detergent residue, even feeling rough and dull.

- Dry skin and hair due to the use of hard water.

- When using hard water to make tea or coffee, a thin layer of scum appears on the surface.

- If you use hard water to make ice, you will find the ice is cloudy and melts quickly.

Types of hard water and effective ways to soften hard water
Currently, hard water is classified with 3 main types: temporary hard water, permanent hard water and total hard water . Each type will have different characteristics and ways of softening water.

Temporary hard water
Temporary hard water is water containing Ca(HCO3)2 and Mg(HCO3)2 salts. This water is temporarily hard because it is easy to soften. These Ca(HCO3)2 and Mg(HCO3)2 salts, when reacted with temperature, will produce precipitated carbonate salts, thereby removing Ca2+ and Mg2+ ions that cause hardness in water.
The simplest way to soften temporary hard water is to boil the water . Besides, you can also use NaOH, Ca(OH)2 , Na2CO3 or Na3PO4 in water to precipitate the compounds present in the water, thereby returning the water with a softer texture.

Everlasting hard water
Permanent hard water is water that contains salts such as MgSO4, MgCl2, CaCl2, CaSO4, which is also the cause of the hardness of water. Unlike temporary hard water, permanent hard water usually cannot be repaired by boiling, because it does not form a precipitate when boiled.
To soften permanent hard water, usually, people use water softening chemicals such as: baking soda (Na2CO3), caustic soda NaOH, barium hydroxide Ba(OH)2, sodium phosphate Na3PO4. In which, the two most popular permanent hard water softeners are Na2CO3 and Na3PO4.

Hard water ingredients
Component hard water is a type of hard water that includes both temporary hardness and permanent hardness, ie containing both Ca(HCO3)2, Mg(HCO3)2 salts and MgCl2, CaCl2, MgSO4, CaSO4 salts.
To soften the component hard water, you can use the same softening methods for permanent hard water and temporary hard water above.
In addition, an effective and less labor-intensive way to soften hard water is to use a water purifier that uses a reverse osmosis RO membrane. RO filtration technology allows to remove almost all soluble and insoluble substances from water, thus effectively softening hard water.

Above is the information about hard water, telltale signs as well as effective ways to soften hard water that lassho.edu.vn shares with you. If you have any questions, please leave information below the article for support!
Thank you for reading this post What is hard water? Recognizing signs and effective ways to soften hard water at Lassho.edu.vn You can comment, see more related articles below and hope to help you with interesting information.
Related Search: