You are viewing the article How to Find the Valence Electrons for Chromium (Cr)? at Lassho.edu.vn you can quickly access the necessary information in the table of contents of the article below.
How to Find the Valence Electrons for Chromium (Cr)?
The 24th element in the periodic table is chromium. The element of group-6 is chromium and its symbol is ‘Cr’. Chromium is a transition element. Therefore, the valence electrons of chromium are determined differently.
This article discusses in detail how to easily calculate the number of valence electrons in chromium. Hopefully, after reading this article you will know in detail about this.
How many protons, electrons and neutrons does a chromium atom have?
The nucleus is located in the center of the atom. Protons and neutrons are located in the nucleus. The atomic number of chromium is 24. The atomic number is the number of protons.
That is, the number of protons in chromium(Cr) is 24. Electrons equal to protons are located in a circular shell outside the nucleus. That is, a chromium atom has a total of twenty-four electrons.
The number of neutrons in an element is obtained from the difference between the number of atomic masses and the number of atoms. That is, neutron number (n) = atomic mass number (A) – atomic number (Z)
We know that the atomic number of chromium is 24 and the atomic mass number is about 52(51.996u). Neutron (n) = 52 – 24 = 28. Therefore, the number of neutrons in chromium is 28.
What are the valence electrons of chromium?
The 1st element in group-6 is chromium. The elements in groups 3-12 are called transition elements. The valence electrons are the total number of electrons in the last orbit. But in the case of transition elements, the valence electrons remain in the inner shell(orbit).
Because the electron configuration of chromium shows that the last electrons enter the d-orbital. The valence electrons determine the properties of the element and participate in the formation of bonds.
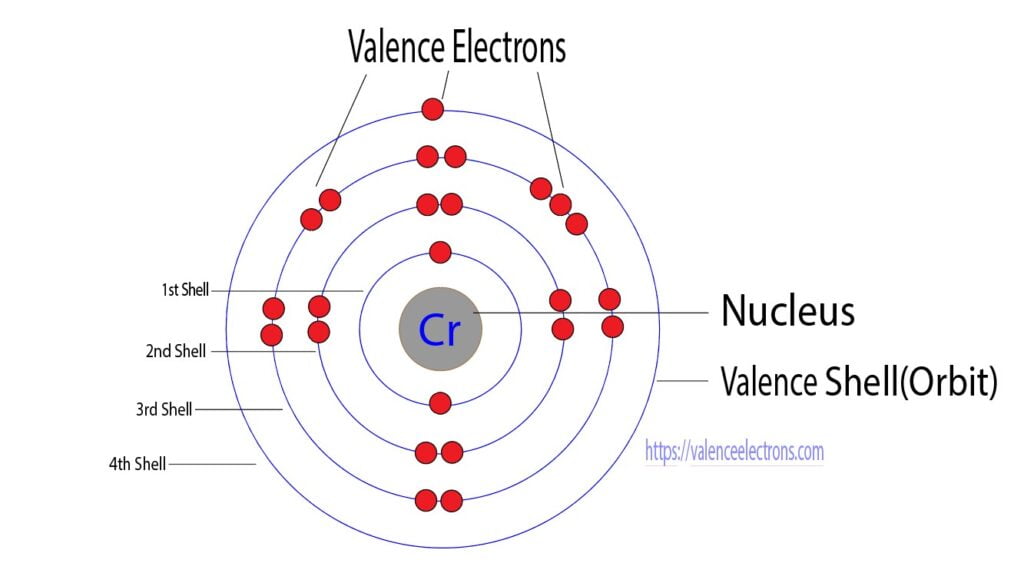
How do you calculate the number of valence electrons in a chromium atom?
The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration.
Knowing the electron configuration in the right way, it is very easy to determine the valence electrons of all elements. The valence electrons of the transition element cannot be determined according to Bohr’s atomic model.
Because the valence electrons of the transition elements are located in the inner shell. However, the valence electron of the transition element can be easily determined according to the Aufbau principle. Now we will learn how to determine the valence electron of chromium.
Step-1: Determining the total number of electrons in chromium
1st we need to know the total number of electrons in the chromium atom. To know the number of electrons, you need to know the number of protons in chromium. And to know the number of protons, you need to know the atomic number of the chromium element.
To know the atomic number we need to take the help of a periodic table. It is necessary to know the atomic number of chromium elements from the periodic table. The atomic number is the number of protons. And electrons equal to protons are located outside the nucleus.
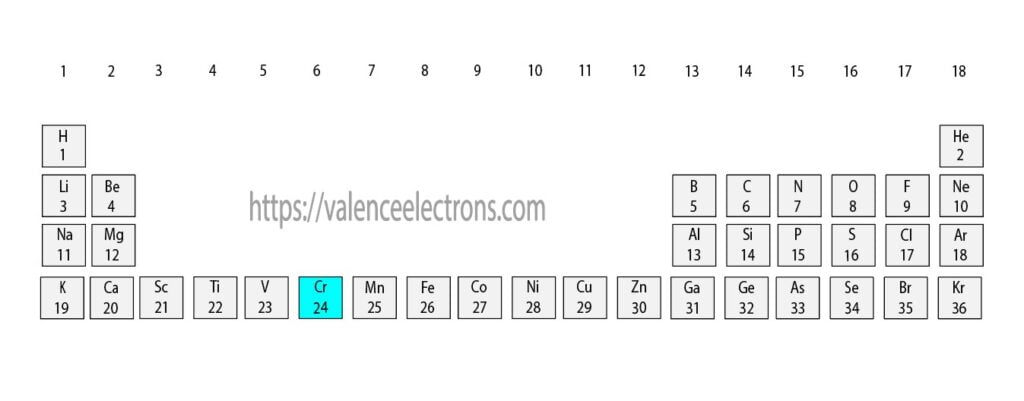
That is, we can finally say that there are electrons equal to the atomic number in the chromium atom. From the periodic table, we see that the atomic number of chromium is 24. That is, the chromium atom has a total of twenty-four electrons.
Step-2: Need to do electron configuration of chromium
Step 2 is very important. In this step, the electrons of chromium have to be arranged. We know that chromium atoms have a total of twenty-four electrons.
The electron configuration of chromium shows that the first shell of chromium has two electrons, the second shell has eight electrons, the 3rd shell has thirteen electrons and the 4th shell has an electron.
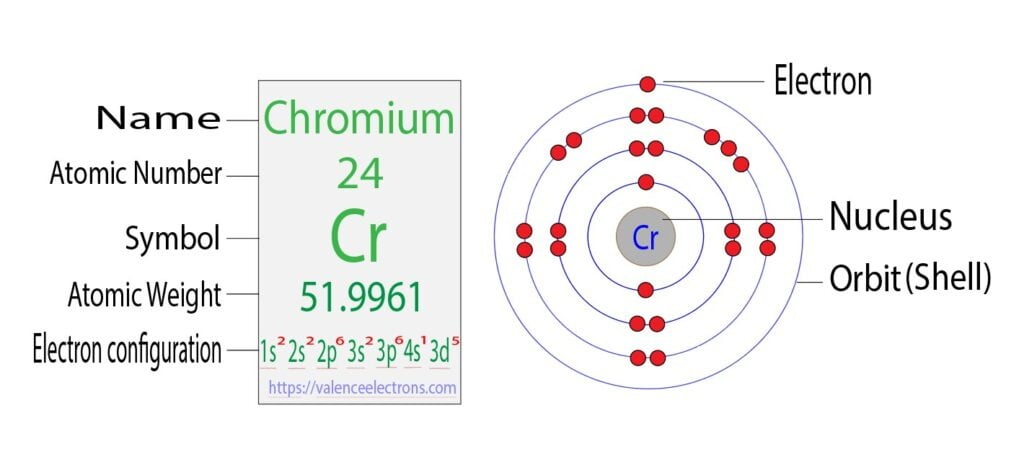
Step-3: Determine the valence shell and calculate the total electrons
The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is called valence electrons.
But the valence electrons of the transition elements are located in the inner orbit. For the transition element, the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom.
The electron configuration of chromium shows that the last shell of chromium has an electron and the d-orbital has a total of five electron. Therefore, the valence electrons of chromium are six.
What is the valency of chromium?
The ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency.
The number of electrons in an unpaired state in the last orbital after the electron configuration of an atom is called the valency of that element.
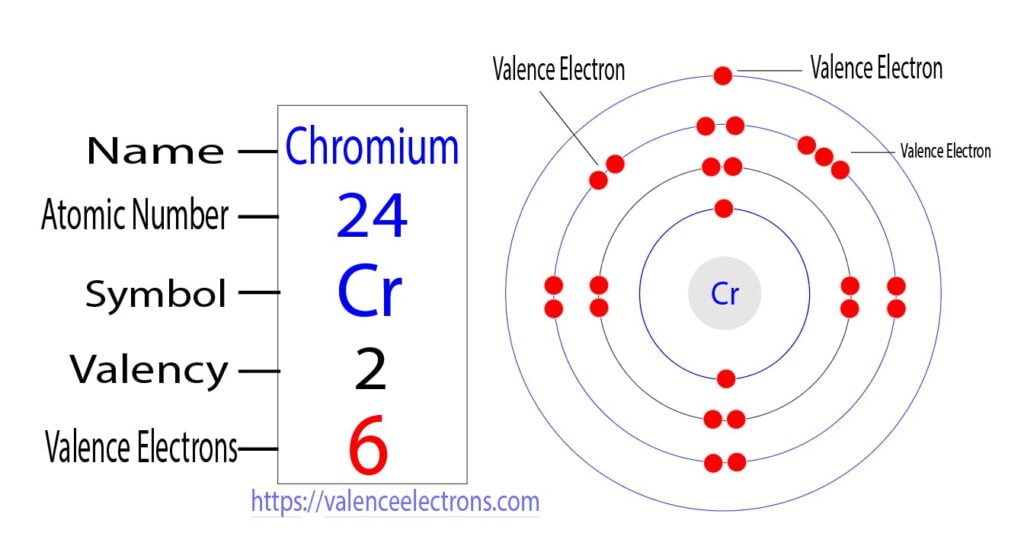
Ground state electron configuration of chromium(Cr) is 1s2 2s2 2p6 3s2 3p6 3dxy2 3dyz2 3dzx1 4s1. Here, the last shell of chromium has two unpaired electrons. Therefore, the valency of chromium is 2.
The valency and oxidation states depend on the bond formation. Chromium 2 and 3 valences are used most of the time. The oxidation states of chromium are +2, +3, and +6.
How many valence electrons does chromium ion(Cr2+,Cr3+) have?
The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating electrons are called cation.
The electron configuration of chromium shows that the last shell of chromium has an electron and the d-orbital has a total of five electrons.
There are two types of chromium ions. The chromium atom exhibits Cr2+ and Cr3+ ions. The chromium atom donates an electron in 4s orbital and an electron in 3d orbital to convert chromium ion(Cr2+).
Cr – 2e– → Cr2+
Here, the electron configuration of chromium ion(Cr2+) is 1s2 2s2 2p6 3s2 3p6 3d4. This electron configuration shows that chromium ion(Cr2+) has three shells and the last shell has twelve electrons. Therefore, chromium ion(Cr2+) has a total of twelve valence electrons.
Cr – 3e– → Cr3+
On the other hand, The electron configuration of chromium ion(Cr3+) is 1s2 2s2 2p6 3s2 3p6 3d3. This electron configuration shows that chromium ion(Cr3+) has three shells and the last shell has eleven electrons. In this case, the valence electrons of the chromium ion(Cr3+) are eleven.
Thank you for reading this post How to Find the Valence Electrons for Chromium (Cr)? at Lassho.edu.vn You can comment, see more related articles below and hope to help you with interesting information.
Related Search:

