You are viewing the article Tasty Mutants: The Invention of the Modern Oyster at Lassho.edu.vn you can quickly access the necessary information in the table of contents of the article below.
Tasty Mutants: The Invention of the Modern Oyster
Genetic innovation, on the half shell
If you slurped down any oysters on the half-shell this summer, you probably didn’t realize they were monsters. Not monsters in the pejorative sense, but man made creatures—the invention of a modern-day Dr. Frankenstein. That Dr. Frankenstein, in this case, is Standish Allen, currently the director of the Aquaculture Genetics and Breeding Technology Center at William & Mary’s Virginia Institute for Marine Science. Over the past three decades, Allen’s patented innovations in oyster culture have transformed this old-fashioned industry. His monster: a sweet, plump morsel called the triploid oyster.
Natural oysters, like humans and most other eukaryotes, are diploid—each of their cells contains two sets of chromosomes, one from each parent. Allen’s innovation has been to create oysters with three sets of chromosomes. The uneven number results in a mostly infertile oyster that, because it doesn’t waste energy producing gametes—eggs and sperm—grows bigger and faster than natural oysters. That means they can be harvested earlier, before they’re affected by the diseases that have laid waste to natural oyster populations in places like the Chesapeake Bay and the estuaries of Normandy.
But the biggest advantage is that these triploids are fat and marketable year-round, even during the warm summer months when natural oysters tend to be unsavory, either because their bodies are comprised mostly of gonads, or because they become thin and watery after spawning.
These characteristics—higher yields and a viable summer product—are why farmed triploids have largely replaced naturally harvested oysters in the nation’s restaurants and oyster bars. Even though most of the oysters produced today are still diploids, the bulk of them are shelled and destined for the soup cannery or some other processed oyster product. That’s especially true for the wild-harvested oysters, which tend to grow in clumps and be misshapen. The lucrative trade in oyster on the half-shell laid out before restaurant goers, though, belongs increasingly to Allen’s fat, beautifully shaped triploids.
And, amazingly, he’s invented them twice.

The first time Allen invented triploid oysters was in the late 1970s, when he was still a masters student at the University of Maine’s Ira Darling Marine Center. The idea, at the time, was to develop products that would bolster Maine’s then nascent aquaculture industry.
“It was very early on in my graduate school career,” Allen says. “I was working with salmon, trying to do basically the same thing with salmon as we later with did oysters—make triploids.”
That’s because induced triploidy had already proven effective at increasing yields in other organisms.
Polyploidy—having more than two sets of chromosomes—is relatively rare in animals, largely restricted to invertebrates and a few amphibians and fishes. In humans, for example, polyploidy is a usually fatal condition (although some specialized somatic cells, like heart muscle and the smooth muscle lining our arteries, are sometimes polyploid). On the other hand, many plants, like blueberries and some redwoods, are naturally polyploid, and agricultural hybridization has induced polyploidy in many others.
In fact, the characteristics of some of the world’s most important crops may derive from the fact that different varieties may have different numbers of chromosomes. The most famous example is wheat. Einkorn wheat, one of the oldest varieties, is a normal, diploid plant, while durum, or macaroni wheat, is tetraploid, and common wheat, or bread wheat, is hexaploid. Botanists induce polyploidy in plants to produce many varieties of seedless fruits, like bananas, grapes, and watermelon. Polyploidy also frequently increases yield.
“In plants,” Allen says, “the benefit of polyploidy is usually a larger plant or fruit. As the DNA content of the cell increases, so does the size of the cell. Therefore, you get what they call ‘polyploid gigantism’. Triploid blueberries, for example, are about twice as large as regular blueberries.”
So Allen hoped to achieve a similar effect on salmon yield. A faster growth rate would also have made it easier to raise salmon in the cold waters of Maine. To do so, triploids looked like a promising approach.
A common way to induce polyploidy in plants is to treat the growth tips of the plant with a toxin called cytochalasin, so Allen began to apply the chemical to the fertilized eggs of his fish subjects. But it didn’t go as planned. “The chemical didn’t work so well on salmon,” he says, “so we decided to give it a try on oysters instead. It worked.”

To understand how it worked, we’re going to have to have a simplified version of “the sex talk”.
Let’s start by pointing out that Allen’s triploids aren’t what we would typically call genetically modified organisms. He doesn’t insert genetic material—let alone genes from another species—into his oysters. Instead, he simply manipulates the basic mechanics of oyster sex.
Sexual reproduction, shorn of its more romantic and prurient elements, is essentially about mixing and combining genetic material, a process that happens both immediately before and during fertilization. As you probably remember from high school biology, this is all part of an intricate intracellular dance called meiosis. Allen just changes up the steps.
Natural meiosis occurs in several highly choreographed stages, each of which happens in rapid succession. In the first stage, even before meiosis technically begins, the genetic material within germ cells—the cells that eventually develop into eggs or sperm—is duplicated. Then, matching chromosomes contributed by that organism’s father and mother are exchanged and assembled into what are essentially four new and unique sets of chromosomes (technically, two pairs of chromatids.) These chromosomes then segregate into two pairs, which are drawn to opposite ends of the cell, allowing the cell to divide into two diploid daughter cells. This division is called meiosis 1.
These daughter cells then divide again—this time without the segregation of genetic material—producing four cells, each now with a single set of chromosomes. That’s called meiosis 2. In most organisms, each of these so-called haploid cells is now a gamete, an egg or sperm cell. Fertilization, the fusing of a haploid egg and a haploid sperm, creates a diploid zygote. Through mitosis—the other form of cellular division that befuddled you in biology class—that zygote eventually develops into an oyster or a human or a plant. In this way, meiosis normally begins and ends with diploids.
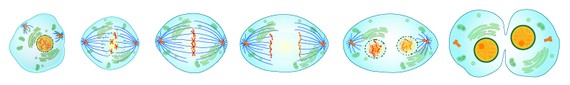
The chemical Allen was applying to salmon and then oysters, Cytochalasin, short-circuits meiosis by preventing the reduction of chromosomes in the daughter cells during meiosis 2. This creates egg and sperm that are diploid, instead of haploid. The timing and dosage of the cytochalasin have to be perfectly controlled, but the result, when a diploid egg is fertilized by a haploid sperm, is a triploid oyster. It was in 1979 when Allen, working at his microscope at the Ira Darling Marine Center, first counted out three chromosomes in one of his oyster gametes. Thus began a new epoch in oyster farming.
Ironically, though, Maine wasn’t ready for Allen’s discovery.
“A discovery,” Allen says, “is really only successful if there’s actual proof of concept in the real world. But in Maine, and on the East Coast in general, most of the oyster industry was still focused on harvesting wild oysters. The industry hadn’t yet developed the large hatchery and breeding infrastructure needed to make triploid production work on a commercial scale.” But while Maine wasn’t ready, there was another oyster market that was. “In the Pacific Northwest, they had all that in place already. They had a large-scale industry putting out millions and millions of oysters a year.”
So, for his Ph.D., Allen moved to the University of Washington where he worked to perfect his chemical triploid process in the large West Coast hatcheries. He hadn’t bothered to patent the chemical techniques he developed at the University of Maine but his new industry partners on the West Coast were eager to protect the intellectual property behind the triploid. Allen’s patent application was ultimately declined, however, because of his previously published work, which meant the technology was already in the public domain. It’s just one more irony in the story that, although Allen didn’t employ genetic engineering to create his triploid, his case did set an important precedent that allowed the patenting of genetically altered animals and ushered in the era of GMO patents.
Allen was eventually awarded a few key patents in the triploid process. His first was actually for a new technique that uses shots of hydrostatic pressure or cold water instead of cytochalasin to interrupt meiosis. By the end of the 1980s, the use of triploids was already widespread among West Coast oyster farmers. “You can think of the late 1980s to the late 1990s as the ‘chemical triploid era’,” Allen says.
But the end of that chemical era was already in sight. First of all, the use of cytochalasin was always a cumbersome process in a hatchery setting. Even in a lab, it was sometimes hit or miss, but on an industrial scale, the survival rate of viable triploids was relatively low. More importantly, the Food and Drug Administration began to put pressure on the industry about its use of a toxin like cytochalasin. By 1989, when Allen finished graduate school and got a job at Haskins Shellfish Research Laboratory at Rutgers University, he was already looking for a new approach to the triploid.
The solution was devilishly elegant: tetraploids–oysters with four sets of chromosomes. The key to this idea was that a tetraploid, because it has an even number of chromosomes, would be fertile. If you crossed a tetraploid oyster with a regular diploid oyster, you could produce an infertile triploid without the use of toxic chemicals. It was brilliant.

But Allen is quick to point out he wasn’t the only one who helped come up with this idea.
“First of all,” he says, “it’s important to give credit to my co-researcher, Ximing Guo, whose name is ahead of mine on this paper. He’s a real scientific researcher; I’m just an old-fashioned bucket biologist.”
It turns out Guo, a post-graduate student from China, the aquaculture capital of the world, was at the University of Washington at the same time Allen was ramping up the chemical production of triploids on the West Coast. “We overlapped by a couple years,” Allen says. “He was always quiet and demure, but he was working away quite confidently on a way to make tetraploids.” Once Guo finished his post-doc in Seattle, Allen lured him to his lab at Rutgers and they set to work on the tetraploid.
Guo’s method was basically an elaboration on the cytochalasin approach, only he was trying to squeeze two extra sets of chromosomes into a regular diploid sperm cell. Once you created the tetraploid, you could breed that oyster with diploids to produce triploids without the use of chemicals. This is the technology behind seedless watermelon.
But it wasn’t easy. “Using these various methods, he was able to make tetraploid embryos from normal eggs, but they were never viable,” Allen said. The problem was that the nuclei of the diploid cells that Guo started with were just too small to accommodate four sets of chromosomes. Allen’s insight was to ask: “What if we started with a larger, triploid cell?”
Nature, it turns out, is full of exceptions. Even though almost all triploids are infertile, every so often you find one that actually can spawn. So, Allen and Guo and the rest of the lab began the search for fertile triploids.
Benoit Eudeline, the research director for Taylor Shellfish, one of the largest oyster hatchery operations in the country, is a former graduate student in Allen’s lab. He remembers the early days of tetraploid research.
“When I was doing my PhD., I had to open hundreds, if not thousands of oysters to find a single fertile triploid,” he says.
In the end, though, Allen’s strategy worked. A few large, fertile triploids were found, and Guo was able to work his magic on them and squeeze the two extra chromosomes into their sperm cells. And, once again, the day came when Allen and Guo were able to verify, this time with a flow cytometer instead of microscope, that they had indeed created a new oyster. The era of the tetraploid had arrived—and today’s tetraploid oysters are all derived from a those few fertile triploids Allen created over a decade ago.
This time, Allen was ready. He made sure he and Guo got the patent on the tetraploid (though, because they were university employees, the patent technically went to Rutgers). Just as important, he and Guo set up a company, aptly called 4-Cs Breeding Technology, to spread the tetraploid gospel. The idea was to license the technology to select hatcheries around the world. Those hatcheries, in turn, can use the tetraploids to produce triploids for the world’s oyster farmers. Right now, commercial hatcheries in Australia and France have the license to produce tetraploids; but the bulk of the tetraploid hatcheries are in the U.S., mostly along the East Coast and the Gulf Coast. The largest producers, though, are still on the West Coast, including the Taylor Shellfish hatchery run by Eudeline.
Interestingly, despite all the benefits of triploids, and the improved production due to the new tetraploid technology, Eudeline thinks triploid production has reached a peak, at least for West Coast growers. The reason, he says, is that triploids can be finicky to grow. While they certainly live up to their hype as a summer product, they don’t always grow faster than their diploid competitors.
“It depends on the location,” Eudeline says. With just the right mix of temperature, salinity and nutrients, the triploid does outperform the diploid. In other cases, the diploid holds up as well, or better. As a consequence, he says, it’s unlikely Taylor will ever go entirely over to triploids, which account for a little over 50 percent of their output. But he adds that part of the reason Taylor still produces so many diploids is simply because of how Taylor operates.
“We have a whole bunch of small farms—dozens of 5-acre, 10-acre, 20-acre farms—and each has its own characteristics. If we were just one big grower, I think we would probably go all triploid. It’s easier to have the whole farm as triploids and not have to switch back and forth between diploids and triploids.”

Even now, it’s instructive to remember that Taylor Shellfish produces hundreds of millions of oysters a year. About half of those are triploids derived from Allen’s tetraploids. It’s also worth noting that other large West Coast oyster producers still grow triploids using Allen’s earlier patented techniques: pressure shots or cold shots.
In some ways, the capital of the tetraploid/triploid world has moved to the Chesapeake Bay. In part, that’s because in 1998 Allen moved his lab to its present location at the Virginia Institute of Marine Sciences at William & Mary. Once again, there’s a touch of irony here, because at the time the industry in the Chesapeake, as it had been in Maine, was very old-fashioned and didn’t really have a hatchery tradition. But the Chesapeake did have one thing that made the transition to triploids plausible: The population of wild oysters had been almost annihilated by a combination of over-harvesting, pollution, and, especially, disease.
This was an ecological crisis for the Chesapeake, but it proved a decisive opportunity for the growth of Allen’s tetraploid/triploid technology. Faced with the loss of the native eastern oyster, Crassostrea virginica, in 2003, the Maryland Department of Natural Resources and the Virginia Marine Resources Commission proposed bringing in a non-native, Crassostrea ariakensis, to replace it.
“That caused all kinds of shit,” says Allen, as various environmental and government organizations clashed with industry about the potential of introducing a new invasive species into the Bay. “But it also gave rise to five or six years of research into whether this was a good idea or not.”
The question, of course, was how to test the viability and relative productivity of the ariakensis without introducing the non-native into the ecosystem. “The answer,” Allen says, “was the mostly infertile triploid. We were compelled to make triploid ariakensis in order to test them; and, as a control, we had to make and test triploids of the native virginica. That put us on track, with money for research, and the incidence of aquaculture in the Chesapeake grew tremendously.”
In the end, Allen notes, the answer to the ariakensis question was “no.” But in the process of testing, the seeds for an aquaculture-based, triploid producing industry were now well established. “The ariakensis actually did a little better than the virginica, but the loser was still pretty good. That kind of convinced a lot of people that you could actually control oysters and make some money at it.”
Today, of course, most of the half-shell oysters that come from the Chesapeake are probably raised in a basket rather than scraped from the bottom. And nearly 90 percent of those are likely triploids. The tetraploid/triploid technology is clearly a commercial as well as an academic success. Which highlights a couple more ironies: Now, just as the growing use of tetraploids represent a potential windfall, the patent is set to run out some time this year. Also, both Allen and Guo, as researchers who advise government agencies on oyster policy, have had to divest their shares in 4C’s to avoid a conflict of interest.
But Allen believes the future of triploid research is bright. Since both its parents are fertile, they can be improved through normal selective breeding. That means it should be possible for future researchers to create triploid varieties to suit very specific climate and environmental niches. That may prove critical, especially on the West Coast, where the industry is under duress from climate change and the resulting ocean acidification. And, as has been demonstrated with some of Allen’s Chesapeake Bay varieties, it should also be possible to breed for disease resistance.
That’s a lot for an old bucket biologist to look forward to.
–>–>
Thank you for reading this post Tasty Mutants: The Invention of the Modern Oyster at Lassho.edu.vn You can comment, see more related articles below and hope to help you with interesting information.
Related Search:

